Our human gastrointestinal tract is harbored with a plethora of microorganisms. Human being has clusters of bacteria present on the surface of the body (skin microbiota), the mouth (oral microbiota), vagina (vaginal microbiota), etc. Human gut microbiota consists of various bacteria, fungi, and viruses present in the human digestive tract.
Benefits Of Gut Microbiota
For Defense
- It defends against harmful microorganisms.
- It degrades toxic compounds.
For Nutrition
- Gut microbiota breaks down dietary fibers and produces important molecules like short-chain fatty acids, which are beneficial.
- Gut microbiota facilitates dietary mineral absorption like magnesium, calcium, and iron.
- Gut microbiota synthesizes some essential vitamins like vitamin K and folate and amino acids.
The gut microbiota provides essential capacities for the fermentation of non-digestible substrates like digestive fibers. The fermentation supports the growth of special microorganisms that produce short-chain fatty acids (SCFA) and gases. The major short-chain fatty acid is acetate, propionate, and butyrate.
General Terms
Eubiosis: microbial balance within the body.
Dysbiosis: It is defined as reducing microbial diversity and a combination of the loss of beneficial bacteria.
Establishment Of Gut Microbiota
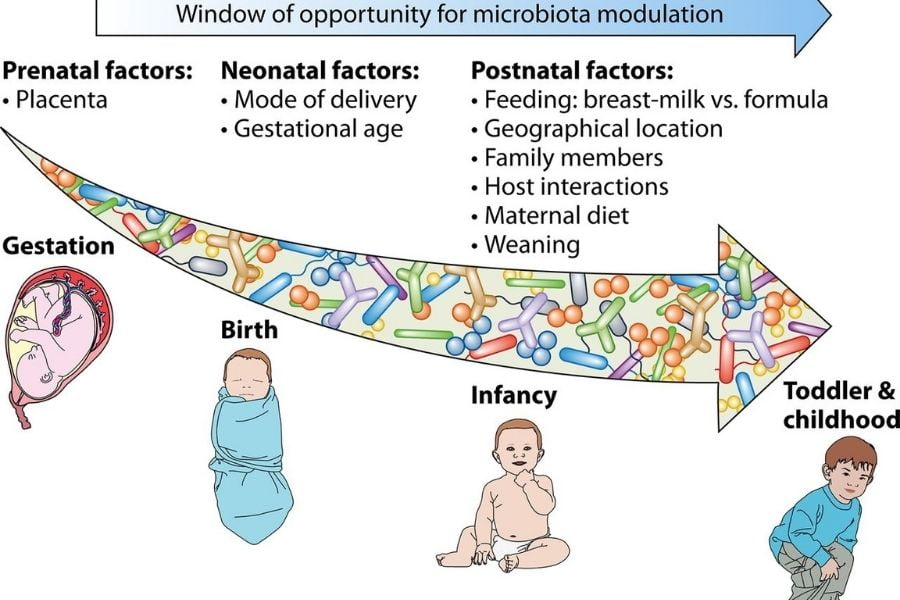
The development of gut microbiota starts during newborns. It is a gradual and dynamic process determined by various factors like delivery modes, prematurity, maternal microbiota, type of feeding, and environmental hygiene.
The initial colonization of the gut lumen is the consequence of the contact and the interaction of both the maternal vaginal and fecal microbes and the surrounding environment. Eventually, the newborn will be colonized with only those microbes to which it is exposed, and that is going to be a permanent community in the neonatal stage.
During the first month of life:
Bifidobacteria, E. coli are the predominant ones, followed by Lactobacillus spp., Bacteroidetes, and gram-positive bacteria, all in similar quantities.
During the first year of life:
The microbial composition of the human intestine is relatively simple and differs from individual to individual. Changes in the gut microbiota are seen in the first year of life due to introducing new food in the infant’s diet. Lactobacillus spp., Bacteroides spp., and clostridia increase in this time, while bifidobacteria and E. coli decrease.
At two years of age:
The microbial community of the infant’s gut reaches a climax with the composition of microbes similarly found in the adult intestine.
Factors Affecting Gut Microbiota
1. Diet

An infant’s diet (breast milk or formula milk) affects gut microbiota. The composition of milk helps in shaping the early gut microbiota. In breast-fed infants, lactobacillus and Bifidobacterium controls the gut microbiota. In formula-fed infants, Enterococcus, Bacteroides, clostridia, and streptococcus controls the gut microbiota. The primary microbiota plays an initial role in the growth of babies.
2. Medication/Drugs Use
Certain medication like antibiotics and proton pump inhibitors alters the gut microbial composition. Proton pump inhibitors reduce acidity in the GI tract, causing high luminal pH, promoting high intestinal bacterial overgrowth. Antibiotic treatment results in rapid loss of diversity and a pronounced community shift.
3. Aging

In old age, the immune system weakens, and also, there are changes in physical activity, digestion process, and nutrients intake that affect the microbial composition. It results in dysbiosis that triggers the proinflammatory state that is linked to health issues.
4. Host Genetics
The host chooses its gut microbiota by producing several molecular signals that control the surface colonized structure by microbiota and influences its composition. Intestinal epithelial cells (IEC) have molecules that include mucus, anti-microbial peptides (AMPs), and immunoglobulin A (IgA), which encourage the growth of some beneficial microbial species and inhibit the others.
Mucus plays a vital role in the large intestine by keeping microorganisms far away from intestinal epithelial cells, shaping gut microbiota, and selecting the most appropriate microbial species for host health.
Anti-microbial peptides (AMPs) play an important role in the small intestine by shaping the gut microbiota. IEC is secreted as the body’s first-line defense against invaders and has an overall effect that kills bacteria, viruses, yeasts, fungi, and even cancer cells.
Immunoglobulin A (IgA): Plasma cells in the intestinal mucosa produces secretory IgA, which covers the bacteria and controls its numbers.
Gut Microbiota’s Role In Health And Disease
Gut microbiota plays an essential role in many gastrointestinal tract diseases.
1. Irritable Bowel Syndrome (IBS)
An irritable bowel syndrome occurs together, including repeated pain in the abdomen and changes in bowel movements, which may be diarrhea or constipation, or both. Recent studies suggest that there is a profile association between dysbiosis and GI tract disease. The IBS patients have fewer Lactobacillus and Bifidobacterium spp than the healthy individuals. Various studies showed that probiotic helps in modifying colonic fermentation and also stabilizes the colonic microbiota. Probiotics showed an improvement in flatulence and abdominal bloating.
Methane (CH4) is a gas produced in the human gut and is produced by anaerobic bacterial fermentation. This gas has been shown to affect the bowel transit velocity, reducing the secretion of serotonin associated with IBS, diverticulosis, and colon cancer.
2. Celiac Disease
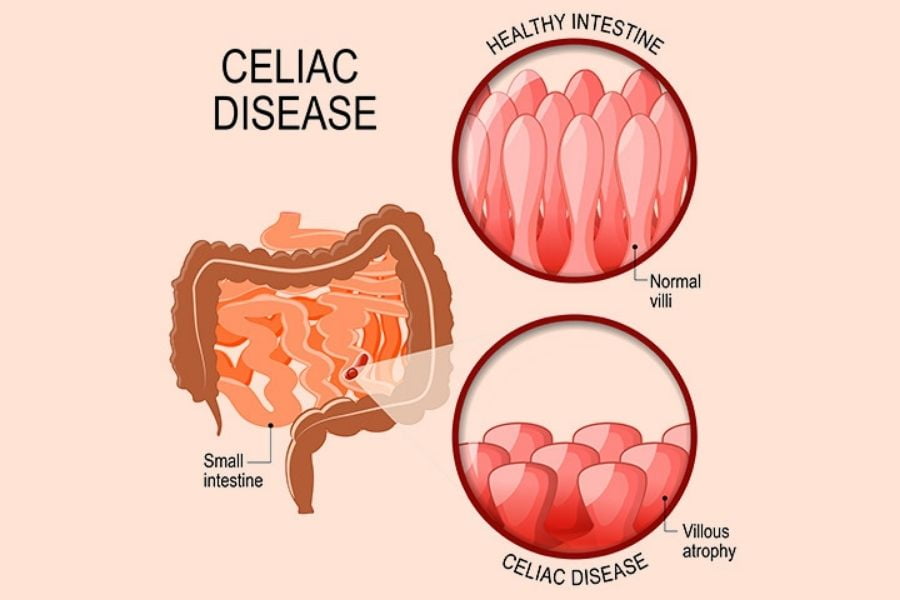
Celiac disease is a chronic inflammatory disorder, primarily affecting the upper small intestine. It is caused by an abnormal immune response to gluten in genetically predisposed subjects with specific HLA-DQ alleles. This enteropathy is more prevalent in children. Gluten is the principal storage protein in wheat, barley, and rye which is poorly digested in the human GI tract. Recent studies have shown that the dysbiosis of intestinal microbiota is linked with celiac disease. The treatment with a gluten-free diet shows improvement in those children suffering from the improved staphylococcus and E. Coli count.
3. Obesity and Type 2 Diabetes

Obesity is a complex syndrome, develops from a prolonged imbalance of energy intake and energy expenditure. Obesity and related disorders such as type II diabetes and metabolic syndrome have become common nowadays. Recent studies generated a new perspective suggesting that gut microbiota might be involved in developing obesity and related disorders. Dietary composition and calorie intake appear to regulate intestinal microbial composition and function.
Hot Topic: Gut Microbiota And Covid-19 Researches
1. Research Study I
SARS Cov-2 primarily is causing lung infection through the binding of ACE2 receptors present on the alveolar epithelial cells. In one of the researches, it was reported that SARS Cov-2 RNA was found in the feces of infected persons. Respiratory virus infection caused perturbation in the gut microbiota. Diet, genetics, and environmental factors play an important role in shaping the gut microbiota, influencing immunity. Gut microbiota is seen to be decreased in the old population, and Covid-19 is seen as fatal in old age groups. Improving gut microbiota profile by personalized nutrition and supplementation has been known to improve immunity. The impact can be minimized in old and immune-compromised patients.
2. Research Study II
In one of the pilot studies, 15 patients who were infected with Covid-19 were taken into consideration. Fecal samples were collected 2-3 times per week from hospitalization until discharge. The disease was categorized as mild, moderate, severe, and critical and compared the microbiome data with those from six subjects with community-acquired pneumonia and 15 healthy individuals (controls). They assessed the gut microbiome profiles with disease severity and changes in fecal shedding SARS-CoV-2.
Results
Patients infected with Covid-19 had significant alterations in fecal microbiomes compared with controls, characterized by enrichment of opportunistic pathogens and depletion of beneficial commensal at the time of hospitalization.
Conclusion
In a pilot study concerning 15 patients with Covid-19, they found persistent alterations in the fecal microbiome during hospitalization time, compared with controls. Fecal microbiota alterations were connected with fecal levels of SARS CoV-2 and Covid-19 severity.